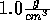 . Insert a noble gas and now slowly decrease the
density in increments of
. Insert a noble gas and now slowly decrease the
density in increments of 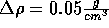 .
.
Background :
When a noble gas is inserted into a stretched water (reduced density) configuration, it has nearly the same effect as when inserted into water at normal density. However, in stretched water, the ``holes'' in water are larger and the stabilizing effect of the noble gas is greater than at normal density.
Start with the NVT ensemble, fix the temperature at 298 K and the density
at 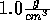 . Insert a noble gas and now slowly decrease the
density in increments of
. Insert a noble gas and now slowly decrease the
density in increments of 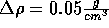 .
.
Questions :