

Next: Vary the number
Up: Simulations with the
Previous: Hydrophobic hydration in
Background :
The solvation (dissolving process)
of a salt molecule is not instantaneous, and not all
different kinds of salt molecules can be dissolved.
When a salt molecule starts to dissolve in water, it first dissociates.
This means that a salt molecule is split by the water
molecules into its two ions (the Wasser program can only simulate
salts consisting of cations from the alkali group (I) and anions from the halide
group (VII)).
After dissociation of the salt into ions, each ion is hydrated --
surrounded by a shell of water molecules.
System settings :
Start with the NVT ensemble, fix the temperature at 298 K and the
density at 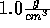 .
Try to insert a salt molecule at an appropriate site.
(If this does not work, lower the density to about
.
Try to insert a salt molecule at an appropriate site.
(If this does not work, lower the density to about 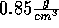 and without waiting for the system to equilibrate, insert a salt molecule.
After insertion, restart the simulation and slowly raise the density to
and without waiting for the system to equilibrate, insert a salt molecule.
After insertion, restart the simulation and slowly raise the density to
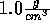 again.)
again.)
Questions :
- Use the insights you have gained to predict what will happen
to the following properties of water after inserting an ionic salt:
the density
the temperature of phase transition
the vapor pressure
- Describe the relationship between the size of salt molecules and
ease (estimated by viewing the potential energy profile) of insertion into the network.
- How do the parameters that you control in the simualtion affect
the ease of insertion?
- Describe the mechanism by which the water molecules separate the
salt molecule into ions?
- Describe the hydration shells around the positive and negative ions.
- What changes occur outside the hydration shell after a salt
molecule dissolves?
- Discuss some of the reasons for the different solubility of different
alkali halides.
- Describe the relationship between the ease of insertion and the dissociation
of a salt molecule.


Next: Vary the number
Up: Simulations with the
Previous: Hydrophobic hydration in
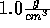 .
Try to insert a salt molecule at an appropriate site.
(If this does not work, lower the density to about
.
Try to insert a salt molecule at an appropriate site.
(If this does not work, lower the density to about 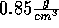 and without waiting for the system to equilibrate, insert a salt molecule.
After insertion, restart the simulation and slowly raise the density to
and without waiting for the system to equilibrate, insert a salt molecule.
After insertion, restart the simulation and slowly raise the density to
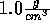 again.)
again.)