

Next: Hydrophobic hydration in
Up: Simulations with the
Previous: Hydrophobic hydration
Background :
When simulating an ion in water, one would assume that a smaller ion is always
easier to insert than a large one. This is the case for water at the density
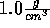 . However, when we stretch water so that its
density is lowered, we
observe an opening of the water structure; the space available
to an ion grows. Very small ions tend to counteract this opening of the
structure because they try to reorient the surrounding water molecules
with the water molecule's charge concentrations towards the charge of the ion and to
pull these reoriented molecules inward, tending to collapse the structure.
A larger ion (the appropriate size depending on the density of the system)
can however stabilize the open structure. This is due to the fact that a
larger ion can ``fill'' the hole much easier without pulling water
molecules towards itself.
The hydration shell of the larger ion now has some similarity with the hydration shell
of a noble gas. The water molecules (depending on the density) do not reorient
towards the ion in the same way for stretched water
as they do for regular water with a density of
. However, when we stretch water so that its
density is lowered, we
observe an opening of the water structure; the space available
to an ion grows. Very small ions tend to counteract this opening of the
structure because they try to reorient the surrounding water molecules
with the water molecule's charge concentrations towards the charge of the ion and to
pull these reoriented molecules inward, tending to collapse the structure.
A larger ion (the appropriate size depending on the density of the system)
can however stabilize the open structure. This is due to the fact that a
larger ion can ``fill'' the hole much easier without pulling water
molecules towards itself.
The hydration shell of the larger ion now has some similarity with the hydration shell
of a noble gas. The water molecules (depending on the density) do not reorient
towards the ion in the same way for stretched water
as they do for regular water with a density of 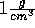 .
Because of this,
the water molecules can still make a small contribution to the
hydrogen bond network.
.
Because of this,
the water molecules can still make a small contribution to the
hydrogen bond network.
System settings :
Start with the NVT ensemble; fix the temperature at 298 K and the density
at 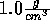 . Insert an ion and now slowly decrease the
density in increments of
. Insert an ion and now slowly decrease the
density in increments of 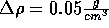 .
.
Questions :
- How does the size of the inserted noble gas influence the water
hydrogen bond network?


Next: Hydrophobic hydration in
Up: Simulations with the
Previous: Hydrophobic hydration
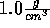 . However, when we stretch water so that its
density is lowered, we
observe an opening of the water structure; the space available
to an ion grows. Very small ions tend to counteract this opening of the
structure because they try to reorient the surrounding water molecules
with the water molecule's charge concentrations towards the charge of the ion and to
pull these reoriented molecules inward, tending to collapse the structure.
A larger ion (the appropriate size depending on the density of the system)
can however stabilize the open structure. This is due to the fact that a
larger ion can ``fill'' the hole much easier without pulling water
molecules towards itself.
The hydration shell of the larger ion now has some similarity with the hydration shell
of a noble gas. The water molecules (depending on the density) do not reorient
towards the ion in the same way for stretched water
as they do for regular water with a density of
. However, when we stretch water so that its
density is lowered, we
observe an opening of the water structure; the space available
to an ion grows. Very small ions tend to counteract this opening of the
structure because they try to reorient the surrounding water molecules
with the water molecule's charge concentrations towards the charge of the ion and to
pull these reoriented molecules inward, tending to collapse the structure.
A larger ion (the appropriate size depending on the density of the system)
can however stabilize the open structure. This is due to the fact that a
larger ion can ``fill'' the hole much easier without pulling water
molecules towards itself.
The hydration shell of the larger ion now has some similarity with the hydration shell
of a noble gas. The water molecules (depending on the density) do not reorient
towards the ion in the same way for stretched water
as they do for regular water with a density of 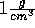 .
Because of this,
the water molecules can still make a small contribution to the
hydrogen bond network.
.
Because of this,
the water molecules can still make a small contribution to the
hydrogen bond network.
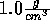 . Insert an ion and now slowly decrease the
density in increments of
. Insert an ion and now slowly decrease the
density in increments of 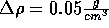 .
.