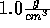 . Insert an ion and view its nearest water molecules.
. Insert an ion and view its nearest water molecules.
Background :
When an ion is inserted into a water configuration, it changes the structure of the hydrogen bond network. A water molecule tends to rotate (reorient) so that its polarized charge concentration faces the opposite charge of the ion. As the water molecules orient themselves towards the ion, they break the hydrogen bonds to their nearest neighbors. The group of water molecules oriented around an ion is called a hydration shell. The orientation of the molecules in the hydration shell results in a net charge on the outside of this shell, a charge of the same sign as that of the ion in the center. The charge on the outside of the hydration shell tends to orient water molecules in the vicinity, leading to a second hydration shell. The result of forming hydration shells is to weaken the structure of the hydrogen bond network. This explains the observation that salt water has a lower freezing point than pure water: Each ion in the liquid has a hydration shell of oriented water molecules around it, which prevents the water molecules from forming the hexagonal structure of ice.
System settings :
Start with the NVT ensemble; fix the temperature at 298 K and the density
at 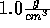 . Insert an ion and view its nearest water molecules.
. Insert an ion and view its nearest water molecules.
Questions :