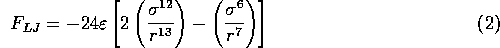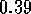 nanometers
for the case shown in Figure 3.5.1.)
nanometers
for the case shown in Figure 3.5.1.)
How does the interaction between water molecules lead to predictions of the large--scale bulk properties of liquid water? To answer this question, we must first describe the interactions between water molecules. The Wasser program uses two such interactions that go by the names Lennard--Jones potential and Coulomb potential. The Lennard--Jones potential is an effective potential that describes the interaction between two uncharged molecules or atoms.
The Lennard--Jones potential is mildly attractive as two uncharged molecules
or atoms approach one another from a distance, but strongly repulsive when
they approach too close. The resulting potential is shown in Figure 3.5.1.
At equilibrium, the pair of atoms or molecules tend
to go toward a separation corresponding
to the minimum of the Lennard--Jones potential (a separation of 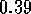 nanometers
for the case shown in Figure 3.5.1.)
nanometers
for the case shown in Figure 3.5.1.)
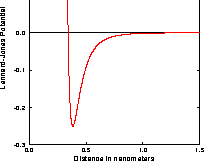
Fig. 3.5.1 The Lennard--Jones potential
The strong close--in repulsion between atoms or molecules is understandable, resulting from mutual deformation of their structures (meaning, one atom cannot diffuse through another.) The mild attraction at larger distances is harder to explain. It results from what is called induced dipole--dipole moment interaction of the particles, described as follows.
Dipole means ``having two poles,'' a positive and a negative one. A dipole is an electrical structure in which charges of equal magnitude but opposite sign are separated along a line (Fig. 3.5.2.) A dipole moment is a measure of the ``strength'' of a dipole. We can increase the dipole moment of a structure either by increasing the magnitude of the positive and negative charges or by increasing their separation. If a molecule with dipole moment is placed in an electric field, the dipole orients along that field.
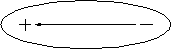
Fig. 3.5.2 A dipole. The direction of the dipole is defined to lie along the arrow that points from the negative charge to the postive charge.
When we look at an uncharged atom, we see a symmetrically distributed (round) electron cloud surrounding its nucleus(Fig. 3.5.3.) As a result, the atom has no dipole moment, because no charges are concentrated in any one direction.

Fig. 3.5.3 An uncharged atom. The positive charge is located in the center, the negative charge (represented by the outer circle) is symmetrically distributed around it.
A particle in a liquid is constantly in motion, undergoing collisions and near--collisions with other particles. When two uncharged particles approach one another, the electron clouds of the competing particles undergo a deformation. During the interaction, each particle does not have a symmetrical electron cloud. As a result each acquires a dipole moment. This is called an induced dipole moment. It lasts for only the short time of near approach, but during this time particles with dipoles are attracted to each other (see Fig. 3.5.4a and Fig. 3.5.4b). This attraction is called the London or Van der Waals force.

Fig. 3.5.4a Two uncharged particles approach one another. The positive charge is located in the the center, the outer ring represents the symmetrical distribution of negative charge around it.
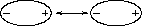
Fig. 3.5.4b After a collision or near collision with another particle, each electron cload undergoes a deformation leading to an induced dipole moment and weak attraction between the particles.
The potential resulting from these attractive and repulsive interactions is called the Lennard--Jones potential and is described by the following equation:
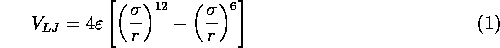
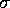 and
and 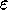 are the specific Lennard--Jones parameters,
different for different interacting particles.
For water, the values of these parameters are:
are the specific Lennard--Jones parameters,
different for different interacting particles.
For water, the values of these parameters are:
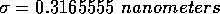 and
and 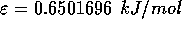 .
When the separation r is very small, the
.
When the separation r is very small, the 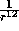 term dominates,
and the potential is strongly positive. Hence the
term dominates,
and the potential is strongly positive. Hence the 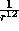 term describes
the short range repulsive potential due to the distortion of the electron
clouds at small separations.
In contrast the
term describes
the short range repulsive potential due to the distortion of the electron
clouds at small separations.
In contrast the 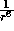 predominates when the separation r increases
in magnitude. Hence the
predominates when the separation r increases
in magnitude. Hence the 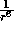 term describes the long--range attractive tail
of the potential between two particles.
term describes the long--range attractive tail
of the potential between two particles.
The Lennard--Jones force between two molecules is given the equation: