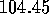 degrees.
degrees.
The hydrogen bond gives water its unique properties. To understand the hydrogen bond, we first take a close look at the water molecule itself. The water molecule is composed of two hydrogen (H) atoms and one oxygen (O) atom (Fig. 3.1.1a). (Figure 3.1.1a may lead to the misconception that the water molecule is flat or planar, which it is not. Rather, it has a tetrahedral structure as shown in Figure 3.1.1b). The two H--atoms in their neutral state each have only one electron. The neutral oxygen atom has six electrons in its outer shell and needs two further electrons to fill all eight places available in this shell (to satisfy the so called octet rule). The H--O bond is a normal covalent bond; each atom contributes one electron to that of its neighbor. The hydrogen atoms each contribute their one and only electron, while the oxygen contributes its two unpaired electrons. Two of these bonds in the structure H--O--H fill up the two vacancies in the oxygen electron structure to complete the octet. Each shared electron pair is concentrated between the two atoms along the bond axis between H and O.
The H--O--H structure has a V--shape (Fig. 3.1.1a).
The angle at the oxygen between the two hydrogens is 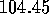 degrees.
degrees.
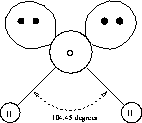
Fig. 3.1.1a A water molecule. The two ``bubbles'' represent the two unshared electron pairs.
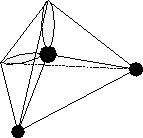
Fig. 3.1.1b The tetrahedral structure of water. The center ball represents the oxygen atom, the two outer black balls the hydrogen atoms, and the two clouds the unpaired electrons.
Now we come to the hydrogen bond. It is what we call a shared electron bond that is a special case of the coordinative covalent bond. The oxygen has two unshared electron pairs that do not contribute to the H--O bonds of the water molecule. These electron pairs are oriented outwards, away from the H--atoms, as shown in Figure 3.1.1. An unshared electron pair can interact with a nearly naked hydrogen nucleus (proton) to form what is called a coordinative covalent bond, or hydrogen bond (Fig. 3.1.2). The hydrogen nucleus appears nearly naked from outside the water molecule, because the hydrogen electron is (mostly) on the inside of the molecule, located along the H--O bond.
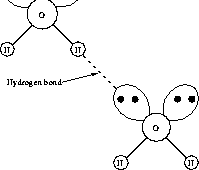
Fig. 3.1.2 A hydrogen bond between two water molecules.
The hydrogen bond is approximately 30 times weaker than a normal covalent bond, because only one of the contributing atoms is supplying electrons to it; the two electrons stay mainly concentrated near the oxygen. Because the hydrogen bond is so weak, it is easily broken. At room temperature, thermal energy is enough to break hydrogen bonds. In liquid water the whole network of hydrogen bonds ``flickers,'' each bond making and breaking again in a millionth of a microsecond. It is this network of flickering hydrogen bonds that gives liquid water its unique properties.
The coordinative covalent bond is not necessarily weak. The nitrogen atom
in the ammonia molecule 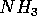 has one lone electron pair. When a hydrogen
ion
has one lone electron pair. When a hydrogen
ion 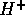 is added to make the ion
is added to make the ion 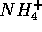 , the four hydrogen atoms
share equally in the resulting bond structure. As a results, the bond energy
between N and each of the four H--atoms is far stronger than the hydrogen
bond between water molecules and is not broken by thermal agitation at room
temperature.
, the four hydrogen atoms
share equally in the resulting bond structure. As a results, the bond energy
between N and each of the four H--atoms is far stronger than the hydrogen
bond between water molecules and is not broken by thermal agitation at room
temperature.