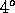 Celsius.
Celsius.
Background :
The solid to liquid phase transition is the melting process of ice.
Any phase transition occurs due to a change in kinetic energy of the
participating particles. If the system is in the solid phase and the
kinetic energy is sufficiently increased, the system changes from solid to liquid.
In the solid phase, the network strives for the lowest energy conformation.
In ice, a water molecule has four nearest neighbors to which it is bonded via
hydrogen bonds (two from its hydrogen atoms and two from the lone electron
pairs on the oxygen.) In the SPC--model, this structure is a result
of the dipole moment and the partial charges on the atoms.
The geometry leads to a rather open hexagonal structure, each
of the four bonds representing a lowered overall energy. When the average kinetic
energy is raised, the additional jostling begins to destroy the open
hexagonal structure.
Paradoxically, this allows the molecules to move closer to
each other, making and breaking hydrogen bonds much more rapidly.
On average, there can now be more than four nearest neighbors at a time, lower energy,
and a higher density in the just--melted liquid system.
When rising temperature increases
average kinetic energy yet further, molecules move through
the liquid so fast that fewer bonds are formed at any one
time, shortening the average time each bond exists. The result is
higher total energy and thus lower density.
Overall, the results is that
at atmospheric pressure the temperature of greatest density is just
above the melting point, at 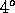 Celsius.
Celsius.
System settings :
Start with the NVT ensemble and with an ice configuration.
The ice configuration can be loaded from the menu, file,
load configuration ice96.dat.
The density can be fixed at 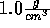 .
Start with temperature of 270 K and slowly raise it.
(Be sure you understand why the density can be fixed at
.
Start with temperature of 270 K and slowly raise it.
(Be sure you understand why the density can be fixed at 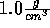 ;
otherwise see the ensemble description.)
Monitor and record the temperature and pressure.
Repeat this, but this time start with the NPT ensemble and gradually increase
the pressure from 0 Mpa in increments of 5 Mpa.
Monitor and record the temperature and density.
;
otherwise see the ensemble description.)
Monitor and record the temperature and pressure.
Repeat this, but this time start with the NPT ensemble and gradually increase
the pressure from 0 Mpa in increments of 5 Mpa.
Monitor and record the temperature and density.
Questions :